Controversial New Alzheimer’s Drug Approved
Aduhelm is the first Alzheimer’s medication to slow the disease, but how the FDA approved the drug and its cost are the target of newly announced congressional hearings
For the first time in its history, the Food and Drug Administration has approved a drug for Alzheimer’s that it describes as a disease-modifying therapy.
In clinical trials at Emory University and elsewhere around the country the medication has been shown to slow the progression of the disease and not just treat its symptoms.
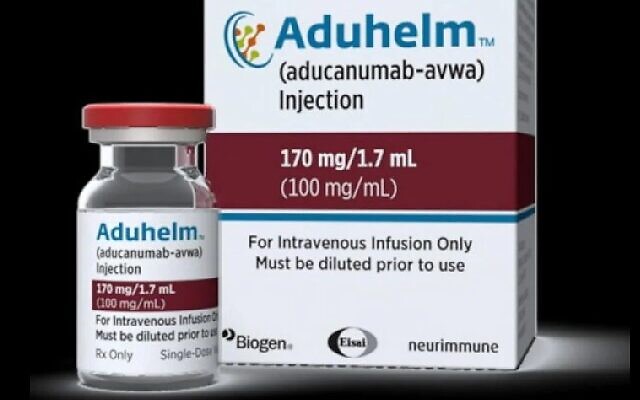
The new drug, called Aduhelm by its manufactures Biogen and Eisai, is also known as Aducanumab.
Approval of the new drug is the first action since 2003 to officially release a new treatment for the devastating disease. But tests have shown that it can cause swelling or hemorrhaging in the brain and, because of that, use of the drug must be accompanied by MRI scans.
Dr. Allan Levey directs the Emory Alzheimer’s Disease Research Center, which took part in the clinical trials for Aduhelm. He is concerned that the medication might be harmful.
“I am most concerned that we need to be very judicious about how the medication is prescribed. There is a significant chance of it doing harm. And if it’s not used really safely and consciously, it’s going to do harm. And so that, to me, is the biggest issue.”
According to Levey, a professor of neurology and head of that Emory Medical School department, the drug has been shown to work only in those patients who are at a very specific stage in the progression of the disease.
“There is no evidence at all that it will work in people that have moderate or advanced stages of Alzheimer’s disease. This is clearly only in a very narrow window where people have symptoms of mild cognitive impairment or very early stages of Alzheimer’s disease, and where experts can do this extra testing that is necessary.” Members of Congress are also concerned about how the drug was approved by the federal agency. On June 30, Congress announced that two congressional committees will hold hearings on the FDA’s action, which has sharply divided Alzheimer researchers in the medical community.
The FDA approval of the drug June 7 occurred despite the objections of many knowledgeable experts and the government agency’s own independent scientific review panel.
However, notwithstanding the controversy, patient advocacy groups such as the Alzheimer’s Association, led by its CEO Harry Johns, welcomed the news of the treatment.
“This is the first FDA-approved drug that delays declines due to Alzheimer’s disease. This means individuals may have more time to actively participate in daily life, have sustained independence and hold on to memories longer.”
The new medication targets what are called amyloids, tangles of protein plaques that develop in the brain of those with the disease, according to a press release about the FDA drug approval.

“The clinical trials for Aduhelm were the first to show that a reduction in these plaques – a hallmark finding in the brain of patients with Alzheimer’s – is expected to lead to a reduction in the clinical decline of this devastating form of dementia,” the press release stated.
But FDA labeling for the drug was especially broad. The label says only that it is “for the treatment of Alzheimer’s disease” and gave no warning of “contraindications” or conditions that might not warrant the use of the drug.
Even more controversial is the cost of the drug, which was set by the manufacturers at $4,312 for each individual infusion or about $56,000 for a year’s supply. Pricing of the drug also is expected to be investigated by Congress.
Estimates by the Kaiser Family Foundation show that even if the new drug was given to only those in the early stages of the disease, the cost to Medicare, which pays for most Alzheimer medications, could come to $29 billion. That is almost 80 percent of what Medicare spent in 2019 for all drugs prescribed by a doctor, KFF reported.
According to Levey that means whoever gets the new drug and whoever doesn’t may be a decision made not just by doctors but by insurers, the final payers.
“I think payers are going to be obviously playing a critical role in determining what the actual practical use is. Payers are going to, hopefully, put some limits on those who they will pay to get the medication.”
Still, Levey sees the development of the new drug – as controversial as it is – as an indication that government spending is beginning to encourage new approaches to treating the disease. He points to the passage 10 years ago of the National Alzheimer’s Project Act that has led to what he described as great progress in developing many promising new therapies.
“There’s been tremendous growth in the Alzheimer’s research enterprise in this country from less than $5 million to over $3 billion a year. This is only the beginning of what’s going to be really far more effective medications than this one.” ì



comments